Import&Sale
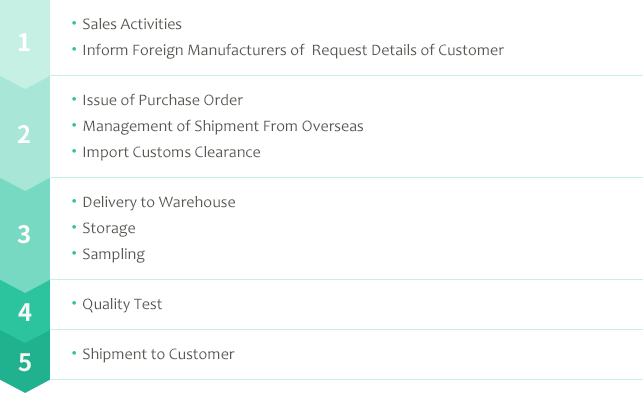
Import&Sale Activities
Our main business is import and sale of generic APIs. We import excellent APIs from foreign manufacturers and sell them to Japanese pharmaceutical manufacturers. To smoothly achieve a supply of safe and reliable APIs at reasonable prices stably to the Japanese market, our sales team works as a pipeline between these two parties.
If foreign API manufacturers want to supply excellent APIs to the Japanese market, we will keep in touch with them and inform them of detailed requests from our customers in Japan. We always assist them to acquire a reputation as reliable manufacturers who can keep such required quality standards and delivery time.
Purchase Order/Shipment Management/Import Customs Clearance
Once our sales staff transfers the requirements from our Japanese customer completely to its foreign manufacturer, we accept a purchase order from our customer and then our Trading Department immediately issues our purchase order to its supplier. Hereafter the department takes care of a daily communication to achieve a stable delivery along with the sales staff.
As soon as the manufacturer dispatches the API we ordered, we transfer the flight details to our forwarder to carry on speedy customs clearance. The condition of package drums is examined carefully twice, after the arrival in Japan and at the time of delivery to our warehouse.
Storage
As a Pharmaceutical Manufacturer License Holder (category: packaging, labeling and storage), we own our storages which meet with GMP (Good Manufacturing Practice) standards in Yokohama and Osaka. We also set special warehouse in Osaka meeting with GMP standards for pharmaceuticals which are designated as hazardous materials under the Service Fire Act. The imported APIs are categorized and stored in strictly controlled clean warehouses until the time of shipment.
Sampling
After the acceptance of imported APIs at our warehouse, we manage their sampling for quality test. Sampling is done in a special sampling room and we particularly use an isolator for API with high activity.
 Isolator(sampling)
Isolator(sampling)Quality Test
As an API importer, we are obliged to perform quality test of imported APIs. Our Pharmaceutical Analysis Center is equipped with two warehouses each in Yokohama and Osaka warehouses. With high-performance equipment, the API samples are strictly tested to perform that they sufficiently meet with the required standards.
 Desktop Scanning Electron Microscope
Desktop Scanning Electron MicroscopeShipment
Once the API meets the standards for shipment as the result of quality test, they are shipped to the customer at their desired time.
However, this is not the end of our business. Hereafter, our Quality Assurance Department will cope with various requests about the quality of APIs from our customers.