Pharmaceutical Analysis
To Prove the Quality of Imported APIs with Accurate Quality Tests
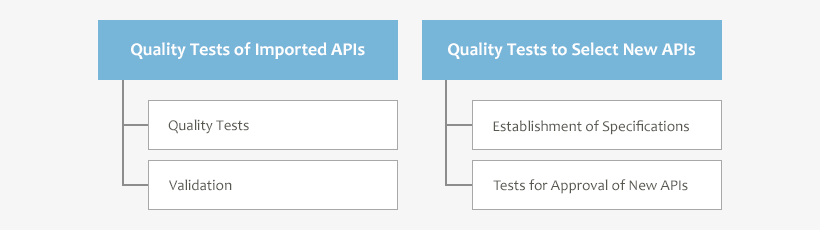
Importers of APIs are obliged to perform quality tests of imported APIs before the shipment to the Japanese pharmaceutical manufacturers.
We have Pharmaceutical Analysis Center equipped with warehouses each in Yokohama and Osaka. Sampling of imported APIs is performed in special sampling rooms. Particularly for APIs with high activity, we use an isolator system to prevent a mix-up and contamination, and keep our staff and environment safe. And after that, we weigh and prepare sample solutions for an instrumental analysis in a safety cabinet.
We also perform validation and quality tests of APIs requested by our customers.
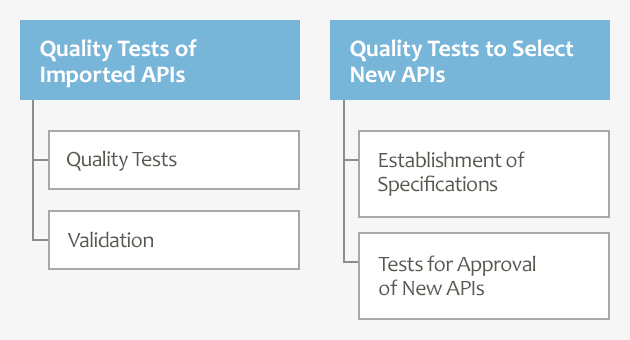
To Perform Quality Tests for the Development of New APIs
Furthermore, in Yokohama, as duties related to the development of APIs, following the requests from our customers, we perform quality tests and establish specifications to select new generic APIs, modify specifications of APIs which have already been used in approved dosage forms when they are added with new dosage forms or partly changed, and perform quality tests for approval of new generic APIs.
What is characteristic about our Pharmaceutical Analysis Center is that it is not only testing facilities of an importer, but also specialized ones which perform various tests concerning generic APIs.